technology
What is Reverse Osmosis?
Reverse osmosis (RO) is a water purification technology that uses a semipermeable membrane to remove larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, acolligative property, that is driven by chemical potential, a thermodynamic parameter. Reverse osmosis can remove many types of molecules and ions from solutions, including bacteria, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as the solvent) to pass freely.
Reverse Osmosis and Removal of Minerals from Drinking Water
OReverse Osmosis will generally remove salt, manganese, iron, flouride, lead, and calcium (Binnie et. al., 2002). Most mineral constituents of water are physically larger than water molecules and they are trapped by the semi-permeable membrane and removed from drinking water when filtered through a RO (AllAboutWater.org, 2004). Meanwhile, consumers are concerned about the removal of minerals from their drinking water.
Reverse Osmosis Remove Minerals
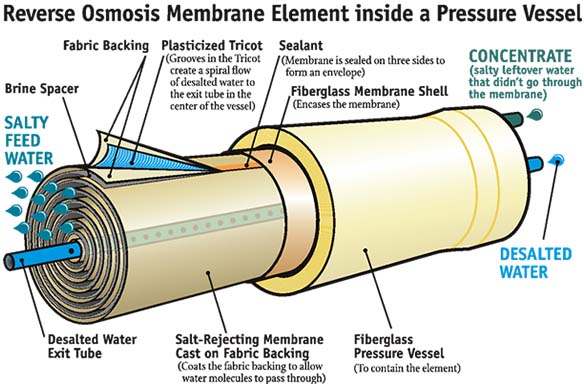
Reverse Osmosis (RO) removed more than 90-99.99% of all the contaminants including minerals from the drinking water supply (seeFigure 1). RO removes minerals because they have larger molecules than water. The subject of minerals and RO created controversy and disagreement among water and health professionals. The World Health Organization (WHO) made clarification that majority of healthy minerals are needed for human body is from food or dietary supplementary sources and not from drinking tap water. In addition, minerals found in water can be harmful to human health. The evidence is strong that calcium and magnesium are essential elements for human body (WQA, 2011). However, its a weak argument to suggest that we should make up this deficiency through water consumption (WQA, 2011). Tap water presents a variety of inorganic minerals which human body has difficulty absorbing (Misner, 2004). Their presence is suspect in a wide array of degenerative diseases, such as hardening of the arteries, arthritis, kidney stones, gall stones, glaucoma, cataracts, hearing loss, emphysema, diabetes, and obesity. What minerals are available, especially in "hard" tap water, are poorly absorbed, or rejected by cellular tissue sites, and, if not evacuated, their presence may cause arterial obstruction, and internal damage (Dennison, 193; Muehling, 1994; Banik, 1989).

Figure 1. Reverse Osmosis Membrane (Source:DOI-BUR, 2009)
Organic Minerals vs. Inorganic Minerals
There are two types of minerals in water, organic and inorganic. Human physiology has a biological affinity for organic minerals. Most organic minerals for our body functions come from dietary plant foods (Misner, 2004). A growing plant converts the inorganic minerals from the soils to a useful organic mineral (Misner, 2004). When an organic mineral (from a plant food) enters the stomach it must attach itself to a specific protein-molecule (chelation) in order to be absorbed, then it gains access to the tissue sites where it is needed (Misner, 2004). Once a plant mineral is divested within the body, it is utilized as a coenzyme for composing body fluids, forming blood and bone cells, and the maintaining of healthy nerve transmission (Balch & Balch 1990).
Reverse Osmosis has Little Affect on Water pH
Water pH levels will automatically change when it is ingested and comes into contact with the food in your stomach (Wise, 2011). Even on an empty stomach, your stomach acid alone is already several times more acidic than RO water (pH 6-8) with a pH level of 2 (Wise, 2011). The human body regulates pH levels constantly to find balance and equilibrium (see Figure 2). Therefore under normal conditions it will always maintain a neutral 7.4 pH balance (Wise, 2011). The healthy body is very robust and it will restore homeostatic pH fairly quickly and easily (Wise 2011). Soft drinks and sports drinks typically have a pH level of 2.5, orange juice has a 3 pH and coffee has a 4 pH level and we drink these beverages all the time without problems (Wise, 2011).Figure 2. Comparison of pH Levels (Source: Wise, 2011)
Figure 2. Comparison of pH Levels (Source: Wise, 2011)